
Let’s look at all the forces on the book: gravity is pulling it vertically down, and there is a “normal force” of the desk surface pushing the book in the direction normal to the desk surface. What does that mean on a molecular level? There must be some sort of attractive force between the book and the desk to hold the book from sliding. Before that, it’s held in place by “static friction”. At a certain angle of tilt, the book begins to slide. Looked at in this second way, it is analogous to thermal conductivity, which is a measure of the rate of transfer of heat from a warm place to a cooler place.įirst, static friction: suppose a book is lying on your desk, and you tilt the desk.

Also, the viscosity of a gas does not depend in its density! These mysteries can only be unraveled at the molecular level, but there the explanations turn out to be quite simple.Īs will become clear later, the coefficient of viscosity \( \eta \) can be viewed in two rather different (but of course consistent) ways: it is a measure of how much heat is generated when faster fluid is flowing by slower fluid, but it is also a measure of the rate of transfer of momentum from the faster stream to the slower stream. Going on to fluids, we’ll give the definition of the coefficient of viscosity for liquids and gases, give some values for different fluids and temperatures, and demonstrate how the microscopic picture can give at least a qualitative understanding of how these values vary: for example, on raising the temperature, the viscosity of liquids decreases, that of gases increases. To begin with, we’ll review the molecular picture of friction between solid surfaces, and the significance of the coefficient of friction \( \mu \) in the familiar equation \( F = \mu N \).
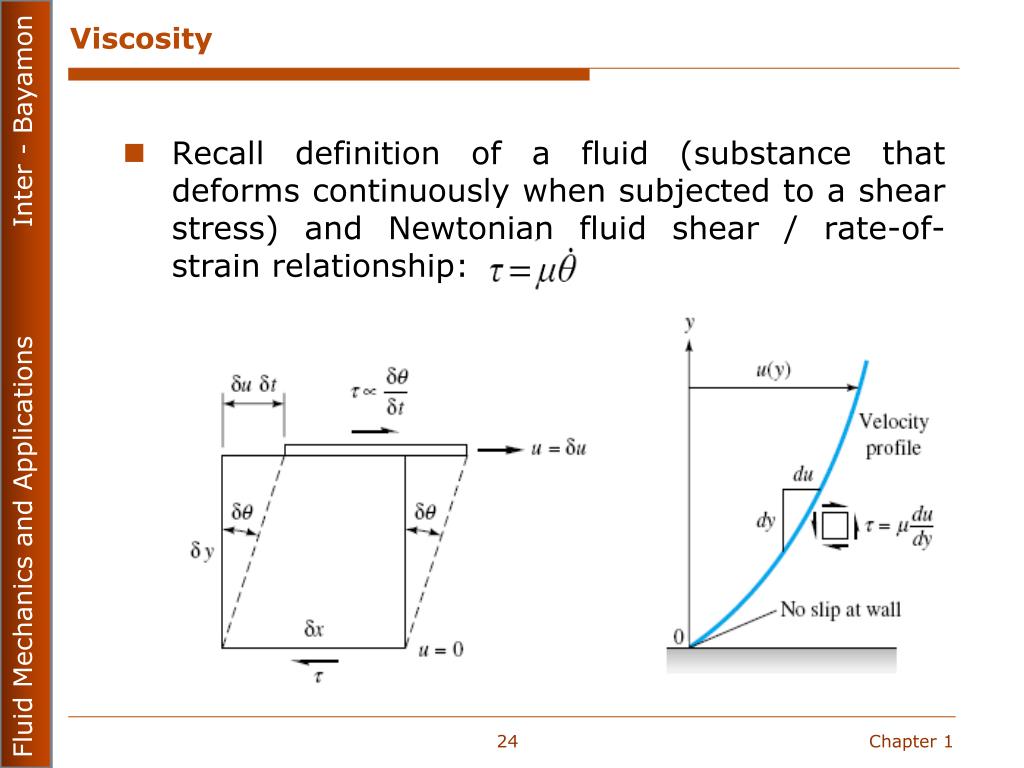
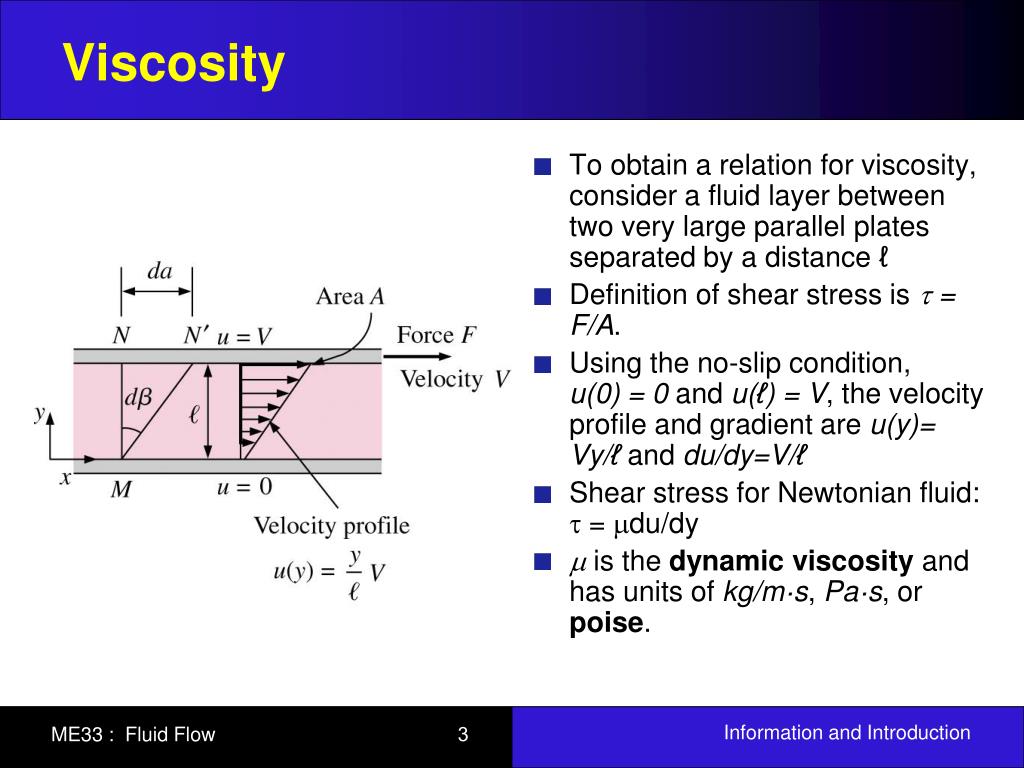
Heat is energy of random motion at the molecular level, so to have any understanding of how this energy transfer takes place, it is essential to have some picture, however crude, of solids and/or liquids sliding past each other as seen on the molecular scale. Like friction between moving solids, viscosity transforms kinetic energy of (macroscopic) motion into heat energy. Viscosity is, essentially, fluid friction.


 0 kommentar(er)
0 kommentar(er)
